Fast adsorption and removal of 2-methyl-4-chlorophenoxy acetic acid from aqueous solution with amine functionalized zirconium metal–organic framework†
Abstract
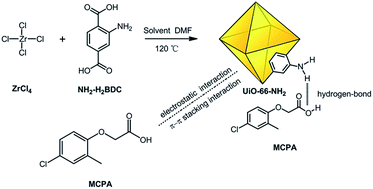
An amino functionalized zirconium-based MOF named UiO-66-NH2 was synthesized and explored as a novel adsorbent for the fast removal of 2-methyl-4-chlorophenoxy acetic acid (MCPA) in aqueous solution. The adsorption process of kinetics, adsorption isotherms, thermodynamics, and adsorbent regeneration were investigated and the effects of key parameters such as adsorbent dosage, pH value and ionic strength on the adsorption of MCPA were also studied. The results showed that the adsorption of MCPA on UiO-66-NH2 was very fast, and most of MCPA were adsorbed in the first 3 min. A pseudo-second-order rate equation effectively described the adsorption kinetics. The adsorption process fits the Langmuir adsorption model well, and the maximum adsorption capacity is 300.3 mg g−1 at 25 °C. The analysis of the adsorption mechanism showed that the hydrogen bond interaction, electrostatic interaction and π–π stacking interaction between the MCPA and UiO-66-NH2 were responsible for the efficient adsorption. Regeneration experiments indicated that the used UiO-66-NH2 was recycled at least six times without significant loss of adsorption capacity. The fast adsorption kinetics, high adsorption capacity, excellent reusability and good chemical stability make UiO-66-NH2 attractive for removal MCPA from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...