Removal of chromium from laboratory wastewater using preparation–adsorption technology with a Mg/Al/Cr layered compound
Abstract
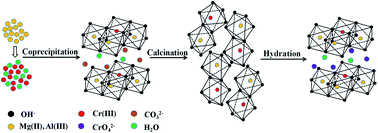
A Mg/Al/Cr layered compound (Mg/Al/Cr(III)–LDH) was prepared using laboratory wastewater containing Cr(III), and the calcined Mg/Al/Cr(III)–LDH compound (Mg/Al/Cr(III)–CLDH) was applied to remove Cr(VI) from laboratory wastewater. The present work simultaneously treats Cr(III) and Cr(VI) pollution using preparation–adsorption technology with the Mg/Al/Cr layered compound. The preparation conditions of Mg/Al/Cr(III)–LDH and the removal process of Cr(VI) were optimized. The maximum adsorption capacity and regenerative properties of Mg/Al/Cr(III)–CLDH were also studied. The physicochemical properties of the materials were analyzed using a combination of X-ray diffraction, FT-IR spectroscopy, TG-DTG, N2 adsorption–desorption, pore size distribution and SEM. The results show that Mg/Al/Cr(III)–LDH performs better when the molar ratio of Mg : Al : Cr(III) is 10 : 4 : 1, the reaction temperature is 45 °C and the aging time is 24 h. The optimal absorption conditions for Cr(VI) are as follows: a pH value of 8–9, a solid–liquid ratio of 1 : 250, and a reaction time of 80 min at room temperature. The maximum adsorption capacity of Mg/Al/Cr(III)–CLDH for Cr(VI) is 237.80 mg g−1. When the concentration of Cr(III) or Cr(VI) in simulated laboratory wastewater is below 300 mg L−1 or 60 mg L−1, the remaining concentration of Cr(III) or Cr(VI) is below to 0.5 mg L−1 after the preparation–adsorption process which is in line with the Chinese National Integrated Wastewater Discharge Standard (GB8978-1996). In addition, Mg/Al/Cr(III)–CLDH could effectively remove Cr(VI) in practical laboratory wastewater, and the treated wastewater could be discharged directly.


 Please wait while we load your content...
Please wait while we load your content...