Oxidation of natural organic matter with processes involving O3, H2O2 and UV light: formation of oxidation and disinfection by-products
Abstract
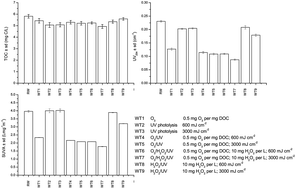
This study investigates the effects of UV photolysis, ozonation and different advanced oxidation processes (O3/UV, H2O2/UV and O3/H2O2/UV) on the oxidation of groundwater natural organic matter (NOM) and by-product formation. Although the investigated treatments only slightly reduce the total organic carbon content (4–15%), the NOM character was changed significantly. The fulvic acid fraction decreased and the content of the hydrophilic acid fraction increased in ozone treated water and even more noticeably in water treated by O3/H2O2/UV. All treatments led to significant increases in polar oxidation by-products such as aldehydes (up to 8 times) and carboxylic acids (up to 34 times), with no clear relationship between the changes in concentrations of these by-products and the addition of H2O2 and the UV dose. Statistical analysis showed a good correlation between carboxylic acids with ozone applications and carboxylic acids and UV254. Trihalomethane and haloacetic acid formation potentials were reduced best (43% for THMFP and 68% for HAAFP) during the O3/H2O2/UV process (0.5 mg O3 per mg DOC; 10 mg H2O2 per L: 600 mL cm−2) using the lower UV dose, and were also well correlated (R = 0.847) during all water treatments. Bromate formation was observed only in the processes involving ozone.

 Please wait while we load your content...
Please wait while we load your content...