An efficient strategy for the synthesis of 5-hydroxymethylfurfural derivative based poly(β-thioether ester) via thiol-Michael addition polymerization†
Abstract
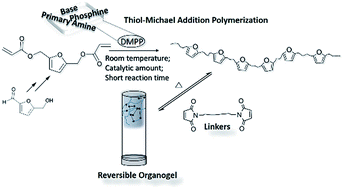
A derivative of 5-hydroxymethylfurfural was synthesized for the thiol-Michael addition reaction. The efficiency of the catalysts (base and nucleophiles) and side reactions during the thiol-Michael addition were investigated. Dimethylphenylphosphine efficiently initiated the thiol-Michael addition polymerization for synthesizing bio-based furan polymers. The product poly(β-thioether ester) showed the potential to be functionalized.

 Please wait while we load your content...
Please wait while we load your content...