Aqueous self-assembly of short hydrophobic peptides containing norbornene amino acid into supramolecular structures with spherical shape†
Abstract
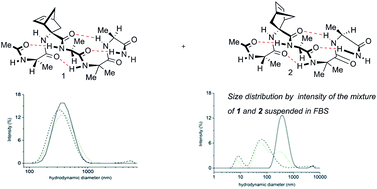
The preparation and self-assembly of short hydrophobic peptides able to solubilize in water through the formation of supramolecular assembly is reported. The two diastereoisomeric pentapeptides AcAla-NRB-Ala-Aib-AlaNH2 1 and 2 containing the two enantiomers of non-proteinogenic norbornene amino acid (NRB) were synthesized in an efficient way and in good yields. They were insoluble in organic solvent, except MeOH and DMSO, but completely soluble in water despite that they are made of hydrophobic amino acids. The formation of a supramolecular assembly in water was assessed by Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS) using 1 and 2 individually or in a mixture. Conformational analysis on the two diastereoisomers, performed in CD3CN, CD3OH and in H2O/D2O, indicated the formation of a stable 310-helix structure for both peptides: the helix structure is more stable in CD3CN and CD3OH than in H2O/D2O where a helix/random coil transition was observed. Apparently, the norbornene moiety plays a role in the stabilization, in fact 1R2R3R-norbornene AA present in peptide 2 induces a more stable secondary structure with respect to the 1S2S3S-isomer present in peptide 1.

 Please wait while we load your content...
Please wait while we load your content...