Kinetics and mechanisms of gas phase reactions of hexenols with ozone†
Abstract
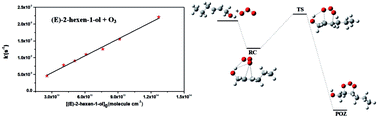
An absolute kinetic study is reported for the reactions of O3 with a series of C6 hexenols, (Z)-2-hexen-1-ol, (Z)-3-hexen-1-ol, (Z)-4-hexen-1-ol, (E)-2-hexen-1-ol, (E)-3-hexen-1-ol, and (E)-4-hexen-1-ol. At 298 K and atmospheric pressure, the rate constants (in units of 10−17 cm3 molecule−1 s−1) were measured to be 7.44 ± 1.03, 5.47 ± 0.71, 7.09 ± 0.91, 16.6 ± 2.2, 6.19 ± 0.72 and 10.5 ± 1.4, respectively. To gain a deeper insight into the reactivity and mechanism, theoretical calculations were also performed for the title reactions with the methods of density functional theory (DFT) and transition-state theory (TST). The geometries, energies, and harmonic vibrational frequencies of each stationary point were obtained at the BH&HLYP/6-31+G(d,p) level of theory. The calculated rate constants are in good agreement with the experimental data, and the reactivity of hexenols with O3 shows a strong dependence on their chemical structure based on the theoretical results. Finally, lifetimes of the C6 hexenols, with respect to their reactions with some important atmospheric oxidants such as O3, OH and NO3 radicals, have also been discussed in the article.

 Please wait while we load your content...
Please wait while we load your content...