Theoretical study on the mechanism of oxidative–extractive desulfurization in imidazolium-based ionic liquid†
Abstract
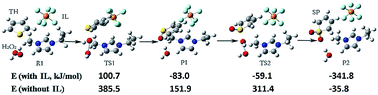
The aim of this paper is to theoretically investigate the mechanism of the oxidative–extractive process for the removal of thiophene (TH) with the assistance of imidazolium-based ionic liquid (IL) by carrying out density functional theory calculations. The results show that IL not only plays an important role in the oxidative process by dramatically decreasing the reaction energy barriers but also exhibits an outstanding performance as an extractive agent. More importantly, we systematically explore the effect of ILs with different alkyl side chains on the imidazolium ring in both the oxidation and extraction process. It is found that with increasing the alkyl chain length from two to six carbon atoms, the energy barriers of the two elementary oxidative steps are reduced by 23% and 29%, respectively, indicating that the longer the alkyl chain, the more easily the reaction takes place. As an extractant, IL shows much stronger interactions with sulphone (SP) than TH owing to the large electronegativity and dipole moment of O atoms in SP. Moreover, natural bond orbital (NBO) analysis illustrates that the cation has a greater binding force with TH or SP than the anion and ionic pairs, and such interaction is slightly decreased as the alkyl chain is lengthened. Our contribution is to provide a thorough explanation for the significantly enhanced oxidative–extractive desulfurization efficiency with the help of IL from the theoretical aspect, which may give a profitable guidance for practical applications.

 Please wait while we load your content...
Please wait while we load your content...