Tuned synthesis and characterizational insight into β-cyclodextrin amended hydrous iron-zirconium hybrid oxide: a promising scavenger of fluoride in aqueous solution†
Abstract
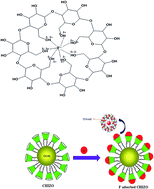
The consumption of water contaminated with fluoride (>1.5 mg L−1) causes serious problems to public health and ultimately leads to skeletal fluorosis. Thus, the development of more efficient fluoride scavenging materials for designing water filters is an immediate task for researchers. β-Cyclodextrin (β-CD) amended hydrous iron–zirconium hybrid oxide (CHIZO), which is a new type of surface modified highly selective composite in organic–inorganic frameworks, is synthesized and characterized using various state of the art analytical tools, and its efficacy on fluoride removal from an aqueous solution is explored. The agglomerated micro structured composite material has no significant fingerprint such as surface appearance in TEM images and is inclined to possess very poor crystallinity. The BET analysis of CHIZO reveals a surface area of 0.2070 m2 g−1 and pore volume of 0.0476 cm3 g−1. The highly pH dependent fluoride adsorption by CHIZO decreases with an increase in pH, and pseudo-second order kinetics control the reaction. The Langmuir isotherm was recognized to be the best fit model to describe the adsorption equilibrium with a significantly higher monolayer adsorption capacity of fluoride (31.35 mg g−1) than the host hydrous Fe–Zr oxide (8.21 mg g−1) at pH ∼7.0 and 303 K. The thermodynamically spontaneous nature of CHIZO is due to the exothermic nature of the reaction. In addition, phosphate and sulphate show an adverse effect on fluoride adsorption. β-CD forms inclusion complexes by taking up fluoride ions from water into its central cavity and the driving forces associated with the complex formation include the release of enthalpy-rich water molecules from its cavity, electrostatic interactions, hydrogen bonding and release of conformational strain. The poor regeneration of the spent adsorbent even in 1.0 M NaOH (below 20%) is probably a consequence of entrapping fluoride inside the cavity of β-CD with hydrogen bonding. It has been found that only 0.9 g of CHIZO is able to reduce the fluoride level to below 1.0 mg L−1 in one-litre of fluoride spiked (5.0 mg L−1) natural water sample. The present study thus reveals that CHIZO could be an efficient adsorbent for fluoride because of its high adsorption capacity and economical viability.

 Please wait while we load your content...
Please wait while we load your content...