A facile one-pot method to synthesise 2-alkylated indole and 2,2′-bis(indolyl)methane derivatives using ketones as electrophiles and their anion sensing ability†
Abstract
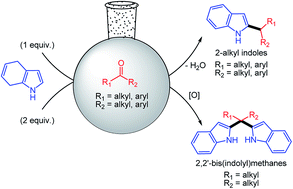
Indole derivatives are of great importance because of their biological activity and application in technology. This study explores the synthesis of 2-alkylated indoles derivatives and 2,2′-bis(indolyl)methanes, and their application in anion sensing. The synthesis of a wide range of 2-alkylated indoles and some 2,2′-bis(indolyl)methanes, which cannot be synthesized by previously reported methods, was for the first time accomplished employing dipole exchange of the indole ring towards electrophilic substitution. Some of the indole derivatives exhibited selective recognition and sensing ability towards F− and HSO4− anions through naked-eye detectable color changes. The sensing details of the indole derivatives were also evaluated using UV-Vis spectroscopy and 1H NMR titration techniques.


 Please wait while we load your content...
Please wait while we load your content...