Toxicity reduction of imidazolium-based ionic liquids by the oxygenation of the alkyl substituent†
Abstract
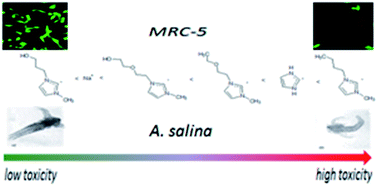
In this work, five different salicylate based ionic liquids were prepared in order to study their toxicity: 1-butyl-3-methylimidazolium salicylate, [bmim][Sal], 1-(4-hydroxy-2-oxybutyl)-3-methylimidazolium salicylate, [OHC2OC2mim][Sal], 1-(3-hydroxypropyl)-3-methylimidazolium salicylate, [OHC3mim][Sal], 1-ethoxyethyl-3-methylimidazolium salicylate, [C2OC2mim][Sal] and imidazolium salicylate [Im][Sal]; aquatic organisms (Artemia salina) and a human non-tumor cell line (normal fetal lung fibroblasts, MRC-5) were also used in the investigation. The introduction of polar groups (in the form of hydroxide and/or ether group) into the alkyl side chain of the imidazolium cation, and their influence on the reduction of the ionic liquid's toxicity, were also demonstrated. The results indicate that the toxicity against A. salina and cytotoxicity against the healthy cell line of lipophobic ionic liquids were significantly lower than for the non-functionalized analogues, and are the same order of magnitude as the reference standard sodium salicylate. These facts open up the possibility of designing new non-toxic ionic liquids that can be used as active pharmaceutical ingredients in liquid form, adjusting only the lipophilicity of the cations by introducing polar oxygen groups into the side alkyl chain of the cation.

 Please wait while we load your content...
Please wait while we load your content...