Copper-catalyzed decarboxylative stereospecific amidation of cinnamic acids with N-fluorobenzenesulfonimide†
Abstract
An efficient copper-catalyzed decarboxylative coupling of cinnamic acids with N-fluorobenzenesulfonimide (NFSI) to give the corresponding substituted (E)-amination products stereospecifically is demonstrated. This new method is efficient and practical, and the corresponding products were obtained in moderate to good yields. The present protocol should broaden the scope of the decarboxylative cross-coupling reactions and provide a useful strategy for the construction of C–N bonds.
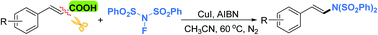

 Please wait while we load your content...
Please wait while we load your content...