Catalytic performance of supported g-C3N4 on MCM-41 in organic dye degradation with peroxymonosulfate†
Abstract
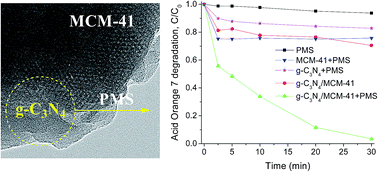
A graphitic carbon nitride-containing MCM-41 catalyst (g-C3N4/MCM-41) was prepared through an in situ thermal approach and characterized by X-ray diffraction, Fourier transform infrared spectroscopy, transmission electron microscopy, N2 adsorption–desorption and X-ray photoelectron spectroscopy. It was found that Acid Orange 7 (AO7) and other five organic dyes can be completely degraded by the g-C3N4/MCM-41 catalyst within 30 min in the presence of peroxymonosulfate (PMS), while g-C3N4 + PMS and MCM-41 + PMS show nearly no activity. A non-radical pathway accompanied by radical generation in PMS activation and AO7 oxidation is proposed. The oxidized carbon species N–C–O on g-C3N4 with strong electron density plays a key role in the reaction, while the large surface area of the catalyst can also efficiently enhance the accessibility of catalytic active sites. Though the reusability of the g-C3N4/MCM-41 catalyst is not very good as that of metal oxide catalysts, its activity can be recovered by KOH treatment. The study can broaden the application of g-C3N4 in the area of wastewater treatment.

 Please wait while we load your content...
Please wait while we load your content...