GSH-responsive polymeric micelles based on the thio–ene reaction for controlled drug release†
Abstract
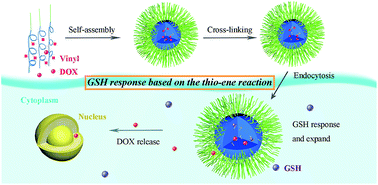
We report a glutathione (GSH) responsive drug carrier that is based on the Michael addition reaction between an olefinic bond and GSH. Amphiphilic copolymers with multi-linear-cyclized and branched structures that contain vinyl groups were successfully synthesized by deactivation enhanced atom transfer radical polymerization (DE-ATRP). The polymers self-assembled into GSH responsive micelles with sizes of 68 to 97 nm and spherical morphology. The core cross-linked micelles were confirmed to greatly decrease doxorubicin (DOX) leakage due to the concentration gradient. Drug release experiments indicated that the drug carrier containing no disulfide has the same responsive ability as a conventional GSH reduction responsive carrier with a similar polymer structure. MTT assays showed that the blank micelles are totally non-cytotoxic, while the DOX-loaded micelles exhibit GSH-dependent behaviour.

 Please wait while we load your content...
Please wait while we load your content...