Combined experimental and theoretical investigations on the encapsulation of nickel(ii)tet-a complex in zeolite Y and its photocatalytic activity†‡
Abstract
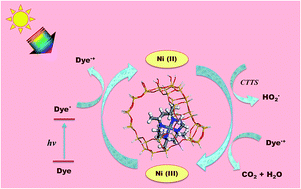
The encapsulation of 5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane [tet-a] nickel(II) in zeolite Y was executed by the “Flexible Ligand method”. The neat and encapsulated complexes were well characterized by DRS UV-Vis, FTIR, powder and single crystal X-ray diffraction studies, ICP-OES, cyclic voltammetry and FESEM. An observed blue shift in the absorption spectrum specified that the structural changes occurred after encapsulation. The energy levels and transition probability of the encapsulated complex varied from the neat metal complex due to the constrained environment of the zeolite. DFT and TDDFT studies were also performed and correlated with the experimental results for both the neat and encapsulated complexes. The obtained experimental UV-Vis spectra were consistent with the theoretically generated UV-Vis spectra. The influence of the zeolite framework on the changes in the structural parameters, energies of the HOMO and LUMO, and global hardness and softness of complex was found via DFT calculations. Fukui functions were used to determine the reactive sites of the complexes. The photocatalytic activity of the encapsulated complex on the degradation of Congo red was carried out under visible light irradiation. The rate constant of the photocatalytic reaction was calculated, and the mechanistic details were proposed.


 Please wait while we load your content...
Please wait while we load your content...