Discovery of a new class of 16-membered (2Z,11Z)-3,11-di(aryl/naphthyl)-1,13-dioxa-5,9-dithia-2,12-diazacyclohexadeca-2,11-dienes as anti-tumor agents†
Abstract
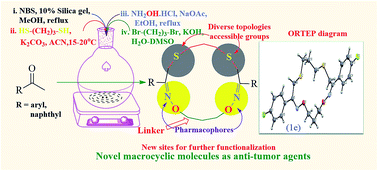
A series of new 16-membered small macrocyclic compounds, (2Z,11Z)-3,11-di(aryl/naphthyl)-1,13-dioxa-5,9-dithia-2,12-diazacyclohexadeca-2,11-dienes (1a–k) were designed and developed by a simple and practical synthetic route from readily available substrates using simple organic transformations. Evaluation of in vitro anti-tumor activities on human triple negative breast cancer cells MDAMB-231 cell lines reveal that the macrocycles, 1a, 1f, 1g, 1i and 1k are promising anti-tumor compounds as evidenced from inhibition of cell migration and proliferation, upregulation of anti-tumor genes p53, MDA7 and TRAIL. The anti-proliferative effect of macrocycles is specific to cancer cells but no cytotoxic effect on normal breast epithelial cells has been observed (MCF10A). The developed synthetic route is free from metals, protecting groups and air-free techniques. The structure of macrocycle (1e) is confirmed by single crystal XRD studies.

 Please wait while we load your content...
Please wait while we load your content...