Nanostructured platinum-free electrocatalysts in alkaline direct alcohol fuel cells: catalyst design, principles and applications
Abstract
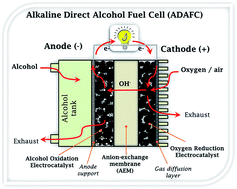
The alkaline direct alcohol fuel cell (ADAFC) is an environmentally friendly electrochemical energy source that can drive a plethora of consumer and portable electronics. Research in ADAFCs has continued to attract major attention due to their several advantages over conventional proton-exchange membrane fuel cells (PEMFC); these include the emergence of anion-exchange membranes (AEM), easy handling of liquid alcohol fuels compared to hydrogen, higher volumetric energy densities of alcohols compared to hydrogen, enhanced reaction kinetics of alcohols and oxygen reduction reaction in alkaline media. Further developments in this field are dependent on improving the performance of nanostructured electrocatalysts and AEMs. This review is an overview of some notable advances made in recent years. Importantly, it provides an excellent insight into the fundamental principles that allow for the intelligent design and synthesis of non-precious metal nanostructured electrocatalysts for the cathode and anode reactions of ADAFCs. This review is an attempt to find answers to questions such as “Why should I use a particular catalyst for the ADAFC?”, “What are the underlying principles that must inform my choice in designing such a catalyst?”, and “What synthesis method(s) or catalyst supports should be considered to prepare catalysts with the appropriate physicochemical properties for high-performance?” The knowledge provided in this review can be applied not only to ADAFCs, but also to several other electrocatalytic systems (such as various other fuel cell systems, electrochemical sensors, and metal–air batteries).

 Please wait while we load your content...
Please wait while we load your content...