Polyaminoacid–doxorubicin prodrug micelles as highly selective therapeutics for targeted cancer therapy†
Abstract
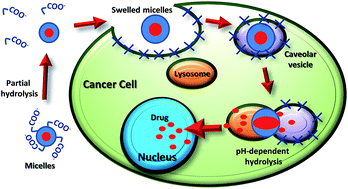
An amphiphilic copolymer carrying high-dose doxorubicin (21% on a weight basis), PHEA–EDA–P,C–Doxo, was prepared by coupling doxorubicin with a biocompatible polyaminoacid through a pH-sensitive spacer. Additional derivatization with 4-pentynoic acid endows it with self-assembling properties by means of π–π stacking. These micelles can be triggered to promptly release drug in lysosomes (∼40% in 12 h) through pH-dependent micelle hydrolysis after uptake. In vitro tests on co-cultures of cancer (MDA-MB 231) and normal (HB-2) breast cells proved that the conjugate was selectively internalized into the former rather than normal cells, exploiting the caveolae-dependent endocytosis pathway, explaining the selective cytotoxic effect toward cancer cells. Intracellular trafficking study of MDA-MB 231 showed that the delivery of the endocytosed drug occurs through the direct fusion of caveosomes with late lysosomes, triggering a massive release in the cytoplasm, bringing about cell death. Dose-effectiveness and mechanistic data indicate that PHEA–EDA–P,C–Doxo is endowed with a distinctive combination of selectivity and pharmacological potency (EC50 13 μM, Emax = 77% and EC50 > 25 μM, Emax = 21% for cancer and healthy cells respectively) that makes it an excellent candidate for future preclinical studies.

 Please wait while we load your content...
Please wait while we load your content...