Oxidative degradation of l-histidine by manganese dioxide (MnO2) nano-colloid in HClO4 medium with/without using TX-100 catalyst: a kinetic approach†
Abstract
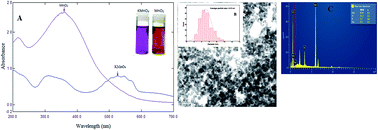
The kinetics of the oxidative degradation of L-histidine (His) in perchloric acid medium by freshly prepared manganese dioxide (MnO2) nano-colloid have been investigated with and without using TX-100 catalyst at a constant temperature of 35 °C. The progress of the oxidation reaction was checked by monitoring the decrease in absorbance of MnO2 spectrophotometrically at 360 nm. The reaction was observed to be first order with respect to [MnO2] and fractional with respect to [His] and [HClO4] in both uncatalysed and TX-100-catalysed paths. The catalytic effect of TX-100 on the oxidation reaction has been explained by the Tuncay model. Protonated L-histidine is bound to TX-100 and forms a [TX-100–His] intermediate complex which further reacts with MnO2 and decomposes into the oxidation products: 2-imidazole acetaldehyde, Mn(II), NH4+ and CO2. On the basis of the kinetics results, a possible reaction mechanism is proposed.

 Please wait while we load your content...
Please wait while we load your content...