Interaction of fluorescent quinolin-2-one and coumarin derivatives including dipeptides with lipid bilayers
Abstract
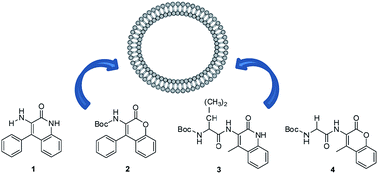
The photophysical properties of two quinolin-2-one and two coumarin derivatives, 3-amino-4-phenylquinolin-2-one (1), N-(tert-butyloxycarbonyl)amino-4-phenylcoumarin (2), and the dipeptides 3 and 4 bearing the quinolin-2-one or the coumarin moiety, respectively, synthesized earlier by some of us, were studied in solvents of varying polarity. Compounds 1 and 2 show a moderate solvent sensitive emission, quinolinone 1 exhibited the highest fluorescence quantum yields (between 0.04 and 0.24). The dipeptide derivatives 3 and 4 present reasonable fluorescence quantum yields in several media, including water (between 0.02 and 0.08). Quinolin-2-one (1 and 3) and coumarin (2 and 4) derivatives were incorporated in lipid vesicles of egg lecithin (egg-PC), DPPC, DPPG and mixed DPPC/DPPG (1 : 1). All compounds are mainly located in the lipid bilayer, feeling the transition between the rigid gel phase and the fluid liquid-crystalline phase. Dynamic light scattering measurements indicated that the liposomes with the encapsulated compounds are generally monodisperse and with hydrodynamic diameters lower than 160 nm, suitable for biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...