Highly water-dispersible magnetite-supported Pd nanoparticles and single atoms as excellent catalysts for Suzuki and hydrogenation reactions†
Abstract
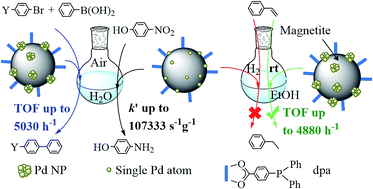
The molecule 4-(diphenylphosphino)benzoic acid (dpa) anchored on the surface of magnetite nanoparticles permits the easy capture of palladium ions that are deposited on the surface of the magnetite nanoparticles after reduction with NaBH4. Unexpectedly, a significant fraction of dpa is removed in this process. Samples of Fe3O4dpa@Pdx containing different Pd loadings (x = 0.1, 0.3, 0.5 and 1.0 wt%) were prepared, and their catalytic efficiency for the Suzuki C–C coupling reaction was studied. The best catalyst was Fe3O4dpa@Pd0.5, which gave the highest TOF published to date for the reaction of bromobenzene with phenylboronic acid in a mixture of ethanol/water (1/1). Interestingly, the same reaction carried out in water also produced excellent yields of the resulting C–C coupling product. The behaviour of other bromide aryl molecules was also investigated. The best catalytic results for the aqueous phase reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) were obtained using Fe3O4dpa@Pd0.1. The presence of Pd SACs (single atom catalysts) seems to be responsible for this performance. In contrast, the same Fe3O4dpa@Pd0.1 catalyst is absolutely inactive for the hydrogenation of styrene in ethanol.

 Please wait while we load your content...
Please wait while we load your content...