Synthesis of 2,4,5-trisubstituted imidazoles, quinoxalines and 1,5-benzodiazepines over an eco-friendly and highly efficient ZrO2–Al2O3 catalyst†
Abstract
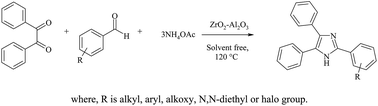
ZrO2–Al2O3 containing 80 mol% of ZrO2 was prepared by solution combustion, impregnation and precipitation methods. All the prepared catalytic materials were characterized by PXRD, NH3-TPD, N2 adsorption isotherms, FTIR, TEM, SEM, EDAX and ICP-OES techniques. These catalytic materials were used in a rapid, simple, versatile and efficient synthesis of 2,4,5-trisubstituted imidazoles under solvent-free conditions at moderate temperature in shorter reaction time (20 min). This is achieved by three-component cyclo-condensation of benzil, aldehydes and ammonium acetate in excellent yields. The derivatives of quinoxalines and 1,5-benzodiazepines were synthesized effectively by cyclo-condensation of 1,2-diamine with α-diketones and carbonyl compound with O-phenylenediamines respectively over ZrO2–Al2O3 at moderate temperature (80 °C) for the reaction time of 20–40 min in presence of ethanol as a solvent. The present method is experimentally simple, non-toxic and involves inexpensive reagents, clean reaction pathways and an eco-friendly catalyst. The catalytic materials used can be easily separated from the reaction mixture and reused for several reaction cycles without much loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...