Synthesis of (−) cephalosporolide E and (+) cephalosporolide F utilizing the vinylogous Mukaiyama type reaction with an α-chloro sulfide†
Abstract
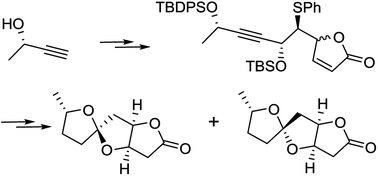
The synthesis of cephalosporolide E and F is described utilizing diastereoselective reduction of a propargylic ketone using a Noyori catalyst to create the C6 carbinol stereogenic center. A vinylogous silylketene acetal addition to an α-chloro sulfide is exploited for stereoselective carbon–carbon bond formation and introduction of the butenolide moiety. Oxidative radical cyclization is utilized for the creation of the [5,5]-spiroketal moiety.

 Please wait while we load your content...
Please wait while we load your content...