Metal-free amidation of carboxylic acids with tertiary amines†
Abstract
A direct amidation of carboxylic acids with tertiary amines could be carried out in the presence of the Ph3P–I2 activator. With an appropriate reagent addition sequence, a range of carboxylic acids including aliphatic, allylic, and aromatic acids could be converted into their corresponding tertiary amides under mild conditions without requirement of metal catalysis.
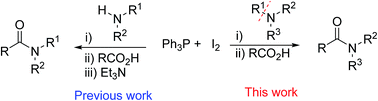

 Please wait while we load your content...
Please wait while we load your content...