One-pot bienzymatic cascade combining decarboxylative aldol reaction and kinetic resolution to synthesize chiral β-hydroxy ketone derivatives†
Abstract
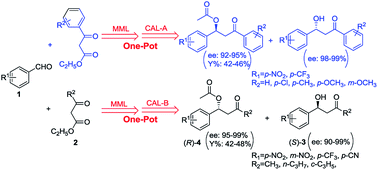
A bienzymatic one-pot sequential cascade for the synthesis of (S)-β-hydroxy ketones and acylated (R)-β-hydroxy ketone derivatives was successfully developed. An immobilized lipase from Mucor miehei (MML) catalysed promiscuous decarboxylative aldol reaction and a lipase A or B from Candida antarctica (CAL-A or CAL-B) catalysed kinetic resolution of racemic β-hydroxy ketone were combined in this one-pot protocol, reducing the purification step between the two reactions. Twelve chiral β-hydroxy ketones and the same number of corresponding acylated derivatives were obtained with excellent ee values and high yields through this method, and the scaling up experiment also worked without apparent loss of reaction rate and stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...