Phosphorus and cobalt co-doped reduced graphene oxide bifunctional electrocatalyst for oxygen reduction and evolution reactions†
Abstract
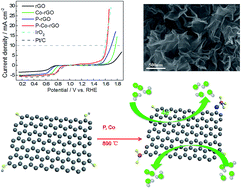
Phosphorus (P) and cobalt (Co) co-doped reduced graphene oxide (P-Co-rGO) has been developed and studied through a facile electrostatic assembly followed by a pyrolysis process. The prepared P-Co-rGO catalyst shows a great enhancement in the electrocatalytic activity and stability towards the oxygen reduction reaction (ORR) in alkaline solution, characterized with a positive onset potential of 0.89 V (vs. RHE), a negative shifting of only about 12.8 mV of the half-wave potential and the closest diffusion limiting current density (−5.5 mA cm−2) as compared to those of the commercial Pt/C (20 wt%). More interestingly, the prepared P-Co-rGO also exhibits excellent catalytic activity and stability for the oxygen evolution reaction (OER), with a low potential of 1.62 V (vs. RHE) at the current density of 10 mA cm−2 and a maximum current density of almost 30 mA cm−2 at 1.66 V (vs. RHE). Specifically, the prepared P-Co-rGO shows much higher activity and stability than the mono-doped reduced graphene oxide either with P or Co, respectively. This could be ascribed to the modification of the charge and spin densities and the edge and defect effects of the rGO after the co-doping of P and Co, thus resulting in a remarkable enhancement of the electrocatalytic properties for both the ORR and OER.


 Please wait while we load your content...
Please wait while we load your content...