Rhodium(ii)-catalyzed direct sulfenylation of diazooxindoles with disulfides†
Abstract
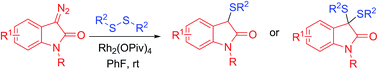
Rhodium(II)-catalyzed direct sulfenylation of diazooxindoles using disulfides as the sulfenylating agent was developed via intermolecular C–S bond formation. This novel protocol provides a rapid synthetic route to 3-alkylthiooxindoles, 3,3-dialkylthiooxindoles, or 3,3-diarylthiooxindoles in moderate to good yield. The transformation is proposed to proceed through sulfur ylide formation followed by S–S bond cleavage and rearrangement.

 Please wait while we load your content...
Please wait while we load your content...