Synthesis of substituted γ- and δ-lactams based on titanocene(iii)-catalysed radical cyclisations of trichloroacetamides†
Abstract
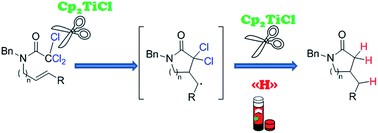
A new procedure for the synthesis of γ- and δ-lactams based on a Cp2TiCl-catalysed cyclisation of trichloroacetamides under mild reaction conditions is reported. Theoretical studies supported the observed regioselectivity in the cyclisations and the mechanism involved in the dehalogenation process.

 Please wait while we load your content...
Please wait while we load your content...