Iron nitrate/TEMPO-catalyzed aerobic oxidative synthesis of quinazolinones from alcohols and 2-aminobenzamides with air as the oxidant†
Abstract
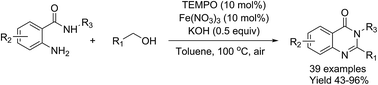
A highly efficient, iron nitrate/TEMPO-catalyzed approach for the synthesis of quinazolinones has been achieved via a one-pot, tandem aerobic oxidative cyclization of primary alcohols with 2-aminobenzamides. This practical reaction tolerates a broad scope of substrates and could afford a variety of desirable products in good to excellent yields with air as the terminal green oxidant. Thus, the present synthetic protocol provides an efficient and concise strategy for the synthesis of quinazolinones.
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...