Facile synthesis of photoactive diaryl(hetaryl)cyclopentenes by ionic hydrogenation†
Abstract
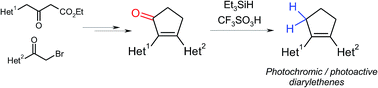
A facile synthetic approach to photoactive diarylethenes comprising a cyclopentene ring as an ethene bridge was developed based on reduction of 2,3-diaryl(hetaryl)cyclopent-2-en-1-ones through an ionic hydrogenation reaction. The method provides access to unsymmetrical photoswitchable diarylethenes containing benzene, thiophene, or azoles (thiazole, oxazole, imidazole) as aromatic moieties in 40–71% yields. Diarylethenes comprising two heterocyclic moieties show typical photochromic properties, with absorption maxima of the photoinduced form in the blue region (yellow photochromes).

 Please wait while we load your content...
Please wait while we load your content...