Design of controlled multi-probe coupled assay via bioinspired signal amplification approach for mercury detection†
Abstract
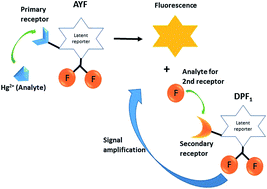
We have designed and synthesized off–on fluorometric probes, 7-(but-3-yn-1-yloxy)-8-(difluoromethyl)-2H-chromen-2-one (AYF) and 7-((4-(tert-butyldiphenylsilyloxy)benzyl)oxy)-8-(difluoromethyl)coumarin (DPF1), by incorporating the concept of biological signal transduction strategy into their molecular design and present their proof-of-concept demonstration for mercury (Hg2+) detection. AYF undergoes a cascade of self-immolative reactions in the presence of Hg2+ and unmasks fluorogenic coumarin 1 and releases two latent fluoride ions. These fluorides initiate the removal of silyl protecting trigger group on DPF1 which directs another cascade of self-immolative reactions. The fluoride ions (amplifiers) are continuously activating the cascade reaction, and, as a result, coumarin 1 progressively accumulates in the reaction medium and the whole process accelerates an amplified signal for Hg2+. For detection of Hg2+, the limit of detection of the two-probe coupling assay (AYF and DPF1) is 0.51 ppb which is more than 2000 times lower than that of AYF alone. Besides, 3-(benzothiazol-2-yl)-4-carbonitrile-7-((4-(tert-butyldiphenylsilyloxy)benzyl)oxy)coumarin (DCC), a long-wavelength probe, was coupled with AYF and DPF1 in order to shift the emission to a higher wavelength region. Negative control studies revealed that the probes undergo controlled and organized sensing pathways as we designed.

 Please wait while we load your content...
Please wait while we load your content...