3-Hydroxyflavone derivatives synthesized by a new simple method as chemosensors for cyanide anions†
Abstract
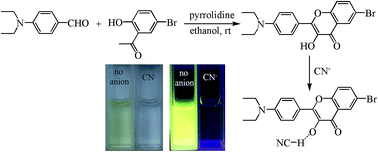
Two novel 3-hydroxyflavone derivatives were synthesized by a one-step simple condensation, cyclization and subsequent oxidation reaction catalyzed by pyrrolidine, which shows great convenience compared with the traditional method. The compounds can recognize cyanide anions at very low concentration with remarkable spectral shift and an obvious color change from yellow to colorless. The bonding mechanism analysis via NMR experiments and mass spectra indicates that cyanide anions induce the deprotonation of the compounds.

 Please wait while we load your content...
Please wait while we load your content...