Structural revision of aristol: a fresh look at the oxidative coupling of thymol under iodination conditions†
Abstract
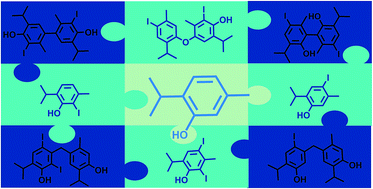
Aristol, an antiseptic drug prepared by iodination of thymol under alkaline conditions, has been on the market since the 1880s. Up until 1951, a myriad of (unlikely) structures were put forward, but none of them were in full agreement with the observed chemical and physical properties of aristol. Today, according to most pharmacopoeias and commercial sources, aristol represents an iodooxybiphenyl derivative; the origin of this structure is unknown. Motivated by these facts, we decided to elucidate the structure of aristol using modern chromatographic and extensive spectrometric techniques (NMR/FTIR/MS/UV), combined with chemical transformations and quantum mechanical calculations. Based on our findings, it was revealed that aristol does not represent a single chemical entity but a complex mixture of a large number of structurally closely related iodinated dehydrodithymol molecules together with (di)iodothymols and unreacted thymol. Five products of oxidative coupling (CAr–CAr, CAr–O–CAr and CAr–CH2–CAr) were successfully isolated in a pure state and fully spectrally characterized: 3,3′-diiodo-5,5′-diisopropyl-2,2′-dimethyl-[1,1′-biphenyl]-4,4′-diol, 5,5′-diiodo-3,3′-diisopropyl-6,6′-dimethyl-[1,1′-biphenyl]-2,2′-diol, 2-iodo-4-(4-iodo-2-isopropyl-5-methylphenoxy)-6-isopropyl-3-methylphenol, 4-(5-hydroxy-2-iodo-4-isopropylbenzyl)-2-isopropyl-5-methylphenol, and 3-(4-hydroxy-5-isopropyl-2-methylbenzyl)-2,4-diiodo-6-isopropylphenol. A lateral meta-coupling (with respect to the phenoxy-radical), CAr–CH2–CAr, previously never reported for oxidative coupling of phenols, was firmly established in two aristol constituents. This provides evidence for a mechanism that involves benzyl radicals unknown to form under these conditions. An additional 16 aristol constituents were identified by the use of a QSPR model (readily available structure-derived descriptors used to predict gas chromatography retention data), in combination with mass spectrometry, directly from the aristol matrix without preparative chromatography. The herein presented results urge a revision of the currently accepted formula (composition) of aristol in both primary and secondary literature.

 Please wait while we load your content...
Please wait while we load your content...