Luminescence and energy transfer in a color tunable CaY4(SiO4)3O:Ce3+, Mn2+, Tb3+ phosphor for application in white LEDs
Abstract
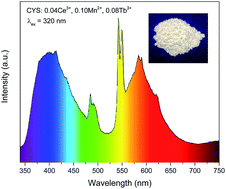
A Ce3+/Mn2+/Tb3+ co-activated CaY4(SiO4)3O phosphor was prepared through high temperature solid state reaction process. By means of dielectric theory of chemical bonding for complex crystals, covalence of chemical bonds in CaY4(SiO4)3O was calculated. Blue emission from Ce3+ in different crystallographic cation sites was discussed quantitatively based on the calculation results. Dual energy transfer of Ce3+ → Mn2+ and Ce3+ → Tb3+ occurs and color tunable emission can be realized by modulation of their relative PL intensity. Energy transfer efficiency has been investigated and the process has been demonstrated to be a resonant type via a dipole–quadrupole mechanism. Critical distance Rc calculated through quenching concentration method and spectral overlap route is 7.23 and 7.55 Å, respectively. As-obtained samples show color tunable emission and the corresponding CIE chromaticity coordinates were given. White light with color coordinates (0.3339, 0.3055) and (0.3553, 0.3338), close to those of the ideal, was realized under lower energy UV light. All results show CaY4(SiO4)3O:Ce3+, Mn2+, Tb3+ phosphor could be a promising candidate for UV chip pumped light emitting diodes.

 Please wait while we load your content...
Please wait while we load your content...