A validated gas chromatography mass spectrometry method for simultaneous determination of cathinone related drug enantiomers in urine and plasma
Abstract
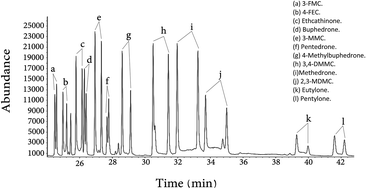
A sensitive and selective method for detection and quantitation of cathinone related compounds using GC-MS has been developed. A chiral derivatization agent, (S)-(−)-N-(trifluoroacetyl)pyrrolidine-2-carbonyl chloride (L-TPC), has been used to achieve enantiomeric separation of some cathinone related drugs. Thirty one synthetic cathinones were separated into their optical enantiomers. Nikethamide was used as an internal standard for cathinone related drug quantitation. The analytical method has been validated in terms of recoveries, reproducibilities, linearities, limits of detection (LOD), and limits of quantitation (LOQ) for all the compounds under investigation. It was found that the LOD of the 12 cathinone derivatives in urine was in the range of 0.1–0.7 ppm and in plasma it was in the range of 0.17–1.33 ppm. The LOQ in urine was in the range of 0.29–2.14 ppm and in plasma it was in the range of 0.50–4.01 ppm.

 Please wait while we load your content...
Please wait while we load your content...