l-proline N-sulfonic acid-functionalized magnetic nanoparticles: a novel and magnetically reusable catalyst for one-pot synthesis of 3,4-dihydropyrimidine-2-(1H)-thiones under solvent-free conditions
Abstract
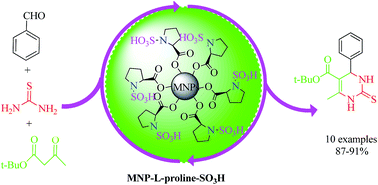
In accordance with the principles of green chemistry, magnetite nanoparticles (MNPs) have been studied in organic research and industry because of their reusability and facile recoverability. In this study, L-proline N-sulfonic acid-functionalized magnetic nanoparticles were prepared as a novel magnetically reusable heterogeneous acid catalyst using a facile process. The catalytic performance of this novel magnetic solid acid catalyst was studied in the synthesis of 3,4-dihydrpyrimidine-2-[1H]thione derivatives in a one-pot three-component condensation reaction of thiourea, aromatic aldehydes, and t-butyl acetoacetate under solvent-free classical heating conditions, with high yield and short reaction times. The structure of the obtained nanoparticles was assessed by scanning electron microscopy (SEM), vibrating sample magnetometry (VSM), thermogravimetry analysis (TGA), X-ray diffraction (XRD), and energy dispersive X-ray analysis (EDXA). The results showed that the synthesized magnetic nanoparticles are superparamagnetic with a size range of 10–20 nm. More importantly, the heterogeneous catalyst could be recovered easily with the assistance of an external magnetic field, and reused many times without significant loss of catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...