Design of recyclable TEMPO derivatives bearing an ionic liquid moiety and N,N-bidentate group for highly efficient Cu(i)-catalyzed conversion of alcohols into aldehydes and imines†
Abstract
Four different types of TEMPO derivatives incorporated with an ionic liquid moiety and N,N-bidentate coordination group (IL–TEMPO-N,N) were prepared. The CuBr/IL–TEMPO-N,N system showed high catalytic activity toward the synthesis of aldehydes and imines via the aerobic oxidation of alcohols in 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4). Both the Cu catalyst and IL–TEMPO-N,N co-catalyst in homogeneous catalytic systems could simultaneously be recovered from the products by extraction using Et2O. The remaining catalyst system in the ionic liquid phase could be reused for several cycles without obvious loss of catalytic activity. Protocols for highly efficient and recyclable aerobic oxidation of alcohols to aldehydes and imines were established.
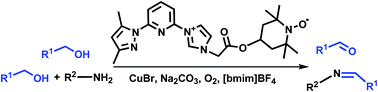

 Please wait while we load your content...
Please wait while we load your content...