Conjugation of biogenic and synthetic polyamines with trypsin and trypsin inhibitor
Abstract
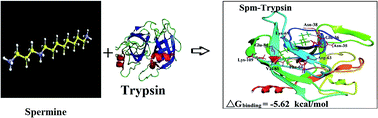
Polyamine–protein conjugates can be used as delivery tools to transport antitumor polyamine analogues. We report the conjugation of trypsin and trypsin inhibitor with biogenic polyamines spermine (spm), spermidine (spmd) and synthetic polyamines 3,7,11,15-tetrazaheptadecane·4HCl (BE-333) in aqueous solution. Multiple spectroscopic methods, thermodynamic parameters and molecular modeling were used to analyse polyamine bindings to trypsin and trypsin inhibitor. Thermodynamic parameters ΔS, ΔH and ΔG showed that polyamines bind protein through H-bonding and van der Waals contacts with trypsin forming more stable conjugates than with the trypsin inhibitor and synthetic polyamines show stronger affinity than biogenic polyamines. Modeling showed that the polyamine–protein interaction is spontaneous and several H-bonding networks stabilize polyamine–protein conjugation.

 Please wait while we load your content...
Please wait while we load your content...