Doxorubicin-loaded redox-responsive amphiphilic dendritic porphyrin conjugates for chemotherapy and photodynamic therapy†
Abstract
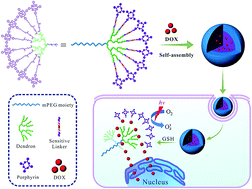
Combination therapy has gained enormous attention due to its high effectiveness in killing cancer cells. Herein, a novel reduction-responsive drug carrier based on amphiphilic dendritic copolymer was constructed for the combination treatment of chemotherapy with photodynamic therapy (PDT). The porphyrin (TPP) disulfide coupled third-generation dendronized poly(ethylene glycol) (PEG-G3-OH) copolymer (TPP-S-S-G3) was synthesized via disulfide linkages between dendritic polyester and porphyrin photosensitizers, which could further self-assemble into spherical micelles in aqueous solution. Compared to the linear TPP-S-S-G0 copolymer, the dendritic TPP-S-S-G3 copolymer could have a higher drug loading content and efficiency. The uptake of doxorubicin (DOX)-loaded TPP-S-S-G3 micelles by MCF-7 cells and intracellular release of porphyrin and DOX was investigated by flow cytometry and CLSM, respectively, and the results exhibited a fast uptake of TPP-S-S-G3 micelles and drug release. Cytotoxicity assay of DOX-loaded TPP-S-S-G3 micelles were evaluated by MTT assay. It indicated that DOX-loaded TPP-S-S-G3 micelles exhibited a higher cellular proliferation inhibition than free DOX, free porphyrin and DOX-loaded linear TPP-S-S-G0 micelles under light irradiation. Thus, the reduction-responsive dendritic copolymer presented the great potential in the combination therapy between chemotherapy and PDT.

 Please wait while we load your content...
Please wait while we load your content...