Iron-catalyzed addition/cyclization cascade of activated alkenes with alcohols: access to carbonyl substituted quinoline-2,4-diones†
Abstract
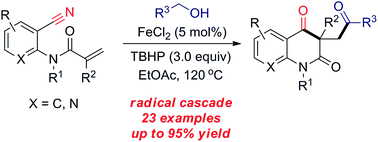
A convenient and efficient iron-catalyzed radical addition/cyclization cascade of o-cyanoarylacrylamides with alcohols is presented for the synthesis of carbonyl substituted quinoline-2,4(1H,3H)-diones in moderate to excellent yields. This transformation exhibits a wide substrate scope and significant functional group tolerance.

 Please wait while we load your content...
Please wait while we load your content...