A novel FTIR-based approach to evaluate the interactions between lignocellulosic inhibitory compounds and their effect on yeast metabolism
Abstract
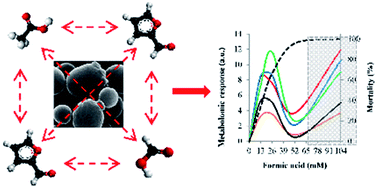
Inhibitors commonly found in lignocellulosic hydrolysates impair yeast metabolism and growth, reducing the productivity of the overall bioethanol production process. FTIR spectroscopy was used to analyze the metabolomic alterations induced by acetic and formic acid, furfural and 5-hydroxymethyl-2-furaldehyde (HMF) on yeast metabolism, using three Saccharomyces cerevisiae strains with different sensitivities. IR spectrum alterations were summarized with synthetic descriptors to rapidly visualize the kinds of molecules displaying the more intense reactions and to evaluate the type of interaction between inhibitors in a mixture, at concentrations close to those found at the industrial scale. The four inhibitors induced different levels of mortality and metabolomic changes. The metabolomic response was proportional to the different strain resistance level, further supporting their original classification. Inhibitor mixtures severely hindered the cell viability with the exception of the lowest concentration tested, which was partially biocidal. Furthermore, for the first time, this study revealed antagonistic interactions exerted by inhibitor mixtures on microbial metabolism, closely strain- and dose-dependent. This confirms that yeast strain resistance to single inhibitors cannot be used to predict behaviour on exposure to mixtures. This finding is worth further studies to explain the underlying antagonistic mechanism and to support the selection of highly tolerant strains.

 Please wait while we load your content...
Please wait while we load your content...