Polycyclic polyprenylated acylphloroglucinols: natural phosphodiesterase-4 inhibitors from Hypericum sampsonii†
Abstract
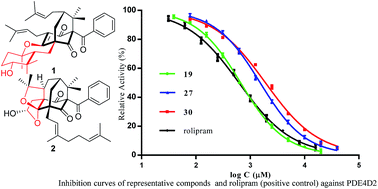
Chemical investigation of the aerial parts of Hypericum sampsonii led to the isolation of seven new polycyclic polyprenylated acylphloroglucinols, hypersampsonones A–G (1–7), together with 23 known analogs (8–30). Their structures including the absolute configurations were elucidated by combined spectroscopic analysis, quantum chemical ECD calculations, and chemical methods. Compound 1 represents an unprecedented cyclocitral monoterpene-coupled bicyclo[3.3.1]nonane skeleton, while 2 features an unusual hexahydrofuro[2,3-b]furan-diepoxy ring system fused in a tricyclo[4.3.1.15,7]undecane skeleton. All the compounds were screened by using tritium-labeled adenosine 3′,5′-cyclic monophosphate ([3H]-cAMP) as substrate for their inhibitory activity against phosphodiesterase-4 (PDE4), which is a drug target for the treatment of asthma and chronic obstructive pulmonary disease. Compounds 1, 18–19, 21, and 25–30 exhibited inhibition with IC50 values less than 10 μM, in which compound 19 represented the most active compound (IC50 = 0.64 μM), being comparable to the positive control, rolipram (IC50 = 0.62 μM).

 Please wait while we load your content...
Please wait while we load your content...