From liquid to thin film: colloidal suspensions for tungsten oxide as an electrode material for Li-ion batteries†
Abstract
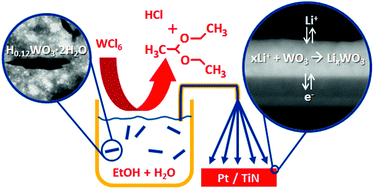
Using a colloidal suspension, tungsten oxide thin films (150 nm) have been prepared via ultrasonic spray deposition using two different current collectors, namely TiN and Pt. First, the precursor chemistry was studied, revealing that the tungsten present is reduced due to the formation of chlorine gas. Due to a dehydrogenation 1,1-diethoxyethane (DEE) and hydrogen chloride (HCl) evolve from the precursor, reducing the chloride content of the precursor. The thin films were annealed at 400 and 500 °C, yielding tetragonal tungsten oxide without the presence of chlorides. Electrochemical analysis indicated that the TiN current collector has a pronounced positive effect on cycling behavior of the WO3 thin film. A higher annealing temperature yields an improved performance, but annealing at temperatures as low as 400 °C also yielded electrochemically active WO3. The current study presents a versatile method to produce electrochemically active tungsten oxide thin films with a high volumetric capacity (640 mA h cm−3) at relatively low temperature to be applied in all-solid-state Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...