Synthetic approaches to nucleopeptides containing all four nucleobases, and nucleic acid-binding studies on a mixed-sequence nucleo-oligolysine†
Abstract
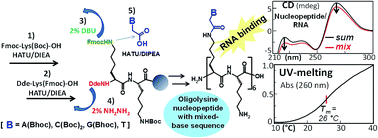
In this article we describe two solid-phase synthetic routes to obtain a nucleo-oligolysine α-peptide containing all four natural nucleobases. The first one is based on the oligomerization of the nucleobase-containing monomers, easily synthesized as herein described. The second strategy has the advantage of avoiding the solution synthesis of the monomeric building blocks, leading to the final nucleopeptide by direct solid-phase couplings of the suitably protected nucleobases with the free amino groups on the growing peptide chain still anchored to the resin. Both strategies are general and can be applied to the synthesis of nucleopeptides having backbones formed by any other diamino acid moiety decorated with the four nucleobases. We also report the CD and UV studies on the hybridization properties of the obtained nucleopeptide, containing all four nucleobases on alternate lysines in the sequence, towards complementary DNA and RNA strands. The nucleo-oligolysine with a mixed-base sequence did not prove to bind complementary DNA, but was able to recognize the complementary RNA forming a complex with a higher melting temperature than that of the corresponding RNA/RNA natural duplex and comparable with that of the analogous PNA/RNA system.

 Please wait while we load your content...
Please wait while we load your content...