Halogen bonding enhances activity in a series of dual 5-HT6/D2 ligands designed in a hybrid bioisostere generation/virtual screening protocol†
Abstract
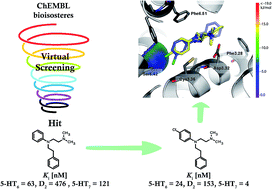
A novel hybrid bioisostere generation/virtual screening method combined with narrowing of chemical space through similarity to compounds that are active at the second target was successfully applied for the development of structurally new dual 5-HT6/D2 receptor ligands. Consequently, a series of derivatives of the found hit 1d (N-[2-(dimethylamino)ethyl]-N-(2-phenylethyl)aniline) was synthesized. The most active 5-HT6/D2 ligands also showed affinity for 5-HT7R and 5-HT2AR. The para-chloroaniline derivative was identified as a potent dual 5-HT6/5-HT7 receptor antagonist (Ki = 24 nM and Kb = 30 nM, Ki = 4 nM and Kb = 1.4 nM, respectively). In the case of halogen-containing compounds, interesting structure–activity relationships were observed at 5-HT6, D2 and 5-HT7 receptors, and the ligand–receptor complexes were subsequently examined using a molecular modelling approach that combined quantum-polarized ligand docking (QPLD) and Molecular-Mechanics-Generalized-Born/Surface Area (MM/GBSA) free-energy calculation, which permitted the identification of putative halogen binding pockets.

 Please wait while we load your content...
Please wait while we load your content...