Three iodocuprate hybrids symmetrically modulated by positional isomers and the chiral conformation of N-benzyl-methylpyridinium†
Abstract
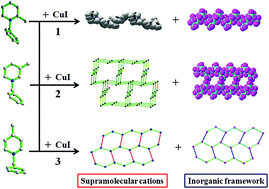
Three iodocuprates, [N-Bz-2-MePy]2[Cu5I7] (1), [N-Bz-3-MePy]2[Cu5I7] (2), and [N-Bz-4-MePy][Cu4I5] (3) (N-Bz-2-MePy+ = N-benzyl-2-methylpyridinium, N-Bz-3-MePy+ = N-benzyl-3-methylpyridinium, N-Bz-4-MePy+ = N-benzyl-4-methylpyridinium) were synthesized and structurally characterized, using benzylated methylpyridinium as a potential conformationally chiral structure-directing agent (SDA). The N-Bz-2-MePy+ cations in 1 are featured in coexisting chiral and achiral conformations, forming a left-handed helical supramolecular chain, while N-Bz-3-MePy+ cations in 2 take on two pairs of mirror-image conformations, aggregating into a racemic supramolecular (10,3)-b net. Comparatively, N-Bz-4-MePy+ cations in 3 possess a couple of mirror-image conformations, forming a supramolecular (6,3) net through π–π and C–H⋯π interactions. Accordingly, 1 and 2 crystallize as conglomerate and racemate packing states, respectively, and 3 possesses no chiral features, which shows a regular directing effect of the positional variation of a methyl group on the construction of iodocuprate frameworks. Furthermore, compounds 2 and 3 exhibit interesting low temperature reversible thermochromism.

 Please wait while we load your content...
Please wait while we load your content...