Novel access to carbonyl and acetylated compounds: the role of the tetra-n-butylammonium bromide/sodium nitrite catalyst†‡
Abstract
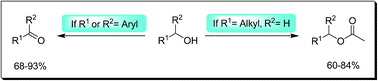
A novel aerobic oxidation of alcohols without the use of any oxidants was developed. An equimolar catalytic mixture of tetra-n-butylammonium bromide and sodium nitrite catalyzes the aerobic selective oxidation of benzylic alcohols under oxidant-free, base-free and metal-free conditions. The mild reaction conditions allow oxidation of a wide range of benzylic alcohols, chemo-selectively to their carbonyl compounds (68–93% isolated yields). More importantly, high selectivity among different kinds of alcohols (aromatic vs. aliphatic alcohols, primary vs. secondary alcohols as well as alcohols having neutral rings vs. electron-deficient rings) is available by this approach. The method surprisingly switched over to be an efficient acetylation approach in the case of aliphatic alcohols without the use of any transition metal, phosphorous or other toxic reagents or any need for using toxic acyl halides, sulfonyl halides, anhydrides, etc. by the use of only acetic acid as a reagent.

 Please wait while we load your content...
Please wait while we load your content...