Enhanced dissolution and oral bioavailability of lurasidone hydrochloride nanosuspensions prepared by antisolvent precipitation–ultrasonication method
Abstract
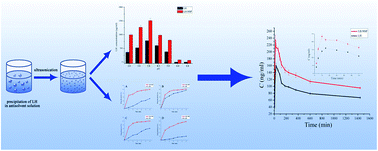
In order to improve the dissolution rate and oral bioavailability of lurasidone hydrochloride (LH), LH nanosuspensions (LH-NSP) were prepared by an antisolvent precipitation–ultrasonication method and characterized in this study. Three important formulation factors including the concentration of LH in the solvent, the amount of sodium dodecyl sulfonate (SDS) and poloxamer 188 (F68) in the antisolvent were optimized by the central composite design response surface methodology. Besides, the impacts of three important process parameters, namely the precipitation temperature, the power input and the duration of ultrasonication, on the particle size and polydispersity index (PDI) of LH-NSP were also investigated. The optimal values of these formulation factors were 0.21% (w/v) LH, 0.06% (w/v) SDS and 0.16% (w/v) F68, respectively, while for the process parameters, the precipitation temperature, power input and duration of ultrasonication were 5 °C, 100 W and 10 min, respectively. The particle size and PDI of the optimized LH-NSP were 124.6 ± 11.9 nm and 0.097 ± 0.0024, respectively. There was no crystalline change in the LH-NSP compared with LH raw material on the basis of powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC) analysis results. With the reduced particle size, the solubility and in vitro dissolution rate of LH in the LH-NSP were significantly improved. Pharmacokinetic studies showed that the Cmax and AUC0–24 of the group with oral administration of the LH-NSP were both 1.5 times higher than that of raw LH.

 Please wait while we load your content...
Please wait while we load your content...