Products, mechanism, and kinetics of OH radical-initiated oxidation degradation of 2,4,4′-trichlorobiphenyl in the atmosphere†
Abstract
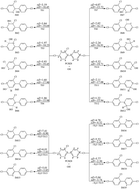
To better understand the behavior, fate and oxidation products of polychlorinated biphenyls (PCBs) in the atmosphere, probing the atmospheric oxidation mechanism and kinetic properties of PCBs is of crucial importance. In this paper, taking 2,4,4′-trichlorobiphenyl (PCB28) as an example, the detailed atmospheric oxidation mechanism initiated by OH radicals was investigated by using quantum chemistry calculations. Studies show that the initial reaction is dominated by OH addition to form the PCB–OH adducts. In the atmosphere, the PCB–OH adducts could further react with O2 either by H-abstraction to form hydroxylated PCBs or by addition to generate peroxy radical intermediates, which subsequently cyclize to bicyclic radicals. Subsequent reactions include O2 addition, NO addition, NO2 elimination and benzene ring breaking. On the basis of the quantum chemical calculation, rate constants of the key elementary reactions at 298 K are calculated. The calculated overall rate constant of PCB28 with OH radicals is 1.25 × 10−12 cm3 per molecule per s with an atmospheric lifetime of 9.3 days. In addition, the effect of substituted chlorine atoms in PCBs to the reaction activity has been discussed.

 Please wait while we load your content...
Please wait while we load your content...