A comparative study on the functionality of S- and P-based lubricant additives by combined first principles and experimental analysis
Abstract
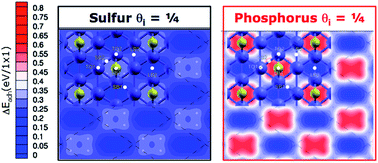
Sulfur and phosphorus are key elements for the functionality of lubricant additives used in extreme pressure applications, such as synchronizer systems in cars. To understand their mechanism of action we combine first principles calculations and gas phase lubrication experiments. The surface spectroscopy analysis performed in situ after the tribological test indicates that iron sulfide (phosphide) is formed by rubbing steel-on-steel in the presence of organo-sulfur (–phosphorus) molecules. We, thus, study the effects of elemental sulfur and phosphorus on the interfacial properties of iron by spin-polarized density functional theory calculations. The results show that both the elements are very effective in reducing the adhesion and shear strength of iron. Sulfur is predicted to be more effective than phosphorus, especially at high pressure. Gas phase lubrication experiments confirm these results, indicating that the friction coefficient of iron-sulphide is lower than that of iron-phosphide and both S and P dramatically reduce the friction of steel-on-steel. These results indicate that the release of elemental sulfur and phosphorus may be the key mechanism to controlling the tribological properties of the metal interface and elucidate that the underling microscopic phenomenon is metal passivation.

 Please wait while we load your content...
Please wait while we load your content...