Steric and chelating ring concerns on the l-lactide polymerization by asymmetric β-diketiminato zinc complexes†
Abstract
A series of new asymmetrical N-aryl-N′-alkyl β-diketimines bearing a pendant pyridyl group (L1H–L4H) were synthesized in a two steps reaction from acetylacetone and the corresponding appropriate amines. The reaction of these β-diketimines (LH) with ZnEt2 afforded the corresponding ethyl zinc complexes. The ethyl zinc complexes were characterized by NMR spectroscopy and single crystal X-ray diffraction to confirm their structure and ligand binding mode. Diffusion-ordered nuclear magnetic resonance spectroscopy (DOSY) experiments yielded diffusion coefficients suggesting that all ethyl zinc complexes are monomeric in solution. All these zinc complexes demonstrate catalytic capabilities towards the ring-opening polymerization of L-lactide. The rate of polymerization depended heavily on the ligand favoring steric bulky aryl substituents (1,3,5-trimethylphenyl) and short pendant arms (pyridylmethyl).
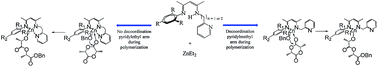

 Please wait while we load your content...
Please wait while we load your content...